By Vidya Rajan, Columnist, The Times
 The deep ocean floor is a desolate place: a desert with frigid waters, crushing pressures and inky darkness. But in the expansive and featureless wasteland are oases populated by bizarre life forms. These oases are located at hydrothermal vents, which were discovered in the mid-1970s but remained difficult to reach and to study for many decades. Recent technological innovations such as Autonomous Unmanned Vehicles (AUVs) and Remotely Operated Vehicles (ROVs) with extended battery life have made more extensive investigation possible, revealing an improbably rich ecosystem with blood-spewing tubeworms, colorless crabs and blind fish. There are two mysteries that studies of life at hydrothermal vents are poised to answer: 1. How life exists in the abyssal depths without the Sun’s energy to power it, and 2. One of the hypotheses of how life evolved on Earth. I will examine each of these in turn.
The deep ocean floor is a desolate place: a desert with frigid waters, crushing pressures and inky darkness. But in the expansive and featureless wasteland are oases populated by bizarre life forms. These oases are located at hydrothermal vents, which were discovered in the mid-1970s but remained difficult to reach and to study for many decades. Recent technological innovations such as Autonomous Unmanned Vehicles (AUVs) and Remotely Operated Vehicles (ROVs) with extended battery life have made more extensive investigation possible, revealing an improbably rich ecosystem with blood-spewing tubeworms, colorless crabs and blind fish. There are two mysteries that studies of life at hydrothermal vents are poised to answer: 1. How life exists in the abyssal depths without the Sun’s energy to power it, and 2. One of the hypotheses of how life evolved on Earth. I will examine each of these in turn.
Hydrothermal vents are molten rock and superheated water-spewing geysers dotted along midocean ridges which form a long seam that loops around the planet like the stitching on a baseball (Figure 1). The seam is nicknamed the “40,000-mile volcano”,[1] because it is where tectonic plates floating on the molten core of the Earth abut. But when two plates collide, their meeting isn’t all handshakes and hail-fellow-well-met. Since we know from Pauli’s Exclusion Principle that two objects cannot occupy the same space at the same time, one of the plates must yield. It does so by diving under the other in regions called “subduction zones” (shown as triangles in Figure 1), and melts into magma in the Earth’s core. To balance this new input, other regions spew out magma, either through volcanoes along the “Pacific Ring of Fire” or more benevolently as new seafloor along the midocean ridge (shown as jagged lines in Figure 1), pushing tectonic plates apart. This conveyer-belt-like “suck-up and spew-out” process also causes shifting of the continents over time: currently the Atlantic Ocean is growing away from Europe by 1.6 inches each year, whereas the Pacific Ocean is shrinking. The “pivot to Asia” is unquestionable, at least geologically.

Figure 1: Tectonic plates bordered by midocean ridges and subduction zones. Figure reproduced from Reference [2].
Chemosynthesis is very much like photosynthesis, but happens with a chemical called hydrogen sulfide, which closely resembles water in structure. Look at the Periodic Table, and you will find sulfur in the same group, right below oxygen. The shared vertical “group” means that sulfur and oxygen have a similar chemical valence, which confers similar reactivity. Oxygen readily bonds with hydrogen to make water, H2O; sulfur bonds readily with hydrogen to make hydrogen sulfide, H2S; and they form the basis of photosynthesis and chemosynthesis, respectively.
In photosynthesis, water is stripped of its energy-rich electrons, releasing oxygen gas and protons (H+) as waste. The high energy electrons are sent along a “wire” and their energy is siphoned off in a process that involves the generation of a proton gradient (described below) to make a remarkable molecule called adenosine triphosphate (ATP). ATP is a rechargeable biological battery. It can be moved around the cell, plugged into different sockets to break or make bonds, or to produce heat.[3] In chemosynthesis, the equivalent reaction with H2S releases elemental sulfur, which is deposited as a solid. But the ATP molecule that is made by chemosynthesis uses a proton (H+) gradient in a manner which is identical to the one by photosynthesis, and its product, ATP, is the source of energy for all biological systems. ATP drives ALL life on Earth.
Chemosynthetic bacteria are the producers of the ocean floor – the equivalent of the chloroplasts of plants. Sometimes these bacteria get eaten as food, but they also live in symbiosis with tubeworms in structures called trophosomes. As a symbiotic partner, the tubeworm helps acquire oxygen and carbon dioxide for chemosynthetic bacteria to grow and produce glucose. A molecule that binds both O and CO2 is hemoglobin (which is chemically a close relative of chlorophyll; Figure 2) and tubeworms use hemoglobin to bind and transport these molecules to the symbiotic bacteria. When tubeworms are injured, they bleed red. Tubeworms and bacteria sustain the shrimp, crabs and fish all clustering around the life-sustaining vent. All these animals are blind. Eyes are expensive to grow and maintain, and useless in the absence of light, and not having them conserves energy. Most of the seabed is empty, like space, cold, dark, and featureless, so these animals don’t really travel.
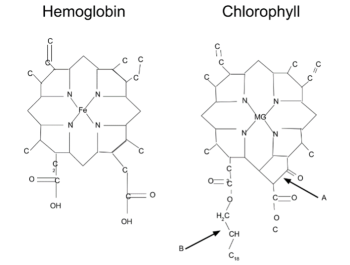
Figure 2: Hemoglobin and chlorophyll, two extremely different molecules when it comes to function, are amazingly similar when it comes to its atomic shape. There are only three major structural differences; a magnesium atom (Mg) in chlorophyll, which is replaced with iron (Fe) in hemoglobin. Additionally, chlorophyll has some extra structures on the bottom right side (A), and an extended hydrocarbon tail on the left (B). These differences cause the chlorophyll molecule to be non-polar, in contrast to the polar hemoglobin molecule. (Note from VR: Hemoglobin is a membrane-located molecule, so is actually pretty non-polar, that is, oil-soluble.) Figure and legend from Reference [4].
Since protons are charged, this sets up a movement of electricity (Figure 3). Once, and apparently once only, in the lifetime of the Earth, an unusual event occurred where lots of carbon molecules had joined with hydrogen ions to form lipids. Because lipids are insoluble in water, they form bubble-like dispersals (like you would get if you shook up some oil in water). One of these events just happened to trap alkaline fluid on the inside of the bubble and acidic seawater on the outside. The proton gradient that this sets up is phenomenal in power terms – a potential difference of 13 million volts/meter, the same as a bolt of lightning! – separated by a thin lipid barrier of about 5 nm thickness.
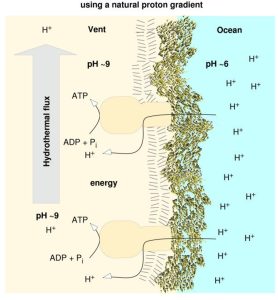
Figure 3: An alkaline hydrothermal vent harbors a natural proton gradient. The flux of hydrothermal effluent maintains an alkaline interior. In the presence of appropriate proteins, this source of energy could, in principle, be tapped. The harnessing of naturally preexisting chemiosmotic gradients before the advent of genetically specified mechanisms to generate such gradients would directly explain why ATP synthases of the F-type (eubacteria) and A-type (archaebacteria) are universal and conserved, but the mechanisms to generate proton gradients are not. Figure and legend from Reference [6].
That “simple beginning” may have been near a hydrothermal vent.
Note: This is one hypothesis of how life may have begun on Earth. I will periodically explore other hypotheses for the origin of life.
References
[1]. Broad, W.J. (2016). The 40,000-Mile Volcano. The New York Times. [online] 12 Jan. Available at: https://www.nytimes.com/2016/01/12/science/midocean-ridges-volcano-underwater.html
[2]. Britannica.com (2024) Available at: https://cdn.britannica.com/s:700×450/65/105565-004-82898B2F.jpg [Accessed 12 Jul. 2024]. Used under fair use copyright for educational purposes
[3]. The brown fat of young babies and hibernating animals contains “uncoupling enzyme” which breaks down ATP to release heat by “non-shivering” thermogenesis. White fat does not contain this enzyme.
[4]. Wikimedia Commons [n.d.] Available at: https://commons.wikimedia.org/wiki/File:Hemoglobin-Chlorophyll.svg [Accessed 12 Jul. 2024]. Used under Creative Commons Attribution-Share Alike 4.0 International license.
[5]. BBC’s The Infinite Monkey Cage Podcast. The Origins of Life. Available at: https://www.bbc.co.uk/sounds/play/b017vsj9
[6]. Martin, W.F. (2011). Early evolution without a tree of life. Biology Direct, 6(1), p.36. doi:https://doi.org/10.1186/1745-6150-6-36. CC BY 2.0 license.
[7]. Short for “Last Common Universal Ancestor”. Weiss, M.C., Preiner, M., Xavier, J.C., Zimorski, V. and Martin, W.F. (2018). The last universal common ancestor between ancient Earth chemistry and the onset of genetics. PLOS Genetics, [online] 14(8), p.e1007518. doi: https://doi.org/10.1371/journal.pgen.1007518
[8]. Gutenberg.org. (2009). On the Origin of Species, 1st Edition by Charles Darwin. [online] Available at: https://www.gutenberg.org/files/1228/1228-h/1228-h.htm




